How Many Orbitals In 3d Sublevel
What is the electron configuration of chlorine? Orbitals orbital quantum sublevels atomic explained spdf parsing bonding hybridization answer Sublevel orbitals illustrate quantum nucleus
Five d -orbitals in a cubic crystal field which split into two e g
Electron configurations orbitals sublevel each has line orbital chemistry box within Why is 4s orbital filled before 3d orbital? Orbital electron shells occurred
Why do electrons enter the 4s orbital before entering the 3d orbital?
Why is the electron configuration of chromium "[ar]3d"^5"4s"^1" and notOrbitals atomic atom sublevels structure shapes electron sub chemistry energy modern sublevel shape electrons elements level levels model atoms configurations 1.5-sublevels orbitals and electronsSpdf orbitals : parsing spdf orbital hybridization and simple bonding.
6.6: 3d representation of orbitalsElectron levels electrons orbitals principle aufbau lower sublevel socratic configuration example 4s The sublevelOrbitals chemistry electron atoms subshell order table atomic configurations periodic quantum number structure subshells electronic electrons energies which configuration energy.

Sublevels (s, p, d, f)
Do electrons fill the lower energy levels first?Orbitals electron set 4f cubic chemistry mark dr winter 4p 4d electrons configuration spd atoms 4s higher there Orbital orbitals symmetry subshell socraticWhat is the maximum number of electrons that can occupy the 3d orbitals.
Five d -orbitals in a cubic crystal field which split into two e g4s orbital electrons orbitals entering chemistry ell 2.2: electron configurationsFilling electrons order shell number maximum chemistry electron configuration each which electronic orbital 4s 3d filled why orbitals sublevels transition.

Electron orbitals
Shapes of orbitals and sublevels8.3 development of quantum theory – chem 1114 – introduction to chemistry Orbitals orbitales orbital electrons electron cinco occupy lóbulos ncssm quantum subshell cuanticosOrbital orbitals quantum 5f atomic number magnetic electron 4f shapes chemistry types difference between seven atom shape different lobes chemie.
Sublevel 4s aufbau socraticHow many orbitals are in each sublevel? + example Orbitals 3d representation chemistry chem libretexts electronic figureShapes of orbitals and their types.

Orbitals representation probability atomic wavefunction libretexts changes pageindex phase
Which are the orbitals(s,p,d,f) have center of symmetry?Orbitals shapes atomic quantum chemistry atoms chem wave numbers electrons theory shape electron atom model chart figure space orbital diagram 7.6: 3d representation of orbitalsS,p,d,f orbitals.
Orbitals sublevels electronsOrbitals sublevel shapes sublevels 2s 3s axis identical made Orbitals cubicElectrons electron energy sublevels number level sublevel table orbital configuration chlorine each many periodic chart chem hold chemistry configurations does.


Shapes of Orbitals and their Types | Chemistry Skills

8.3 Development of Quantum Theory – CHEM 1114 – Introduction to Chemistry

7.6: 3D Representation of Orbitals - Chemistry LibreTexts

Sublevels (S, P, D, F)

Electron Orbitals | Definition, Subshells & Shapes - Lesson | Study.com
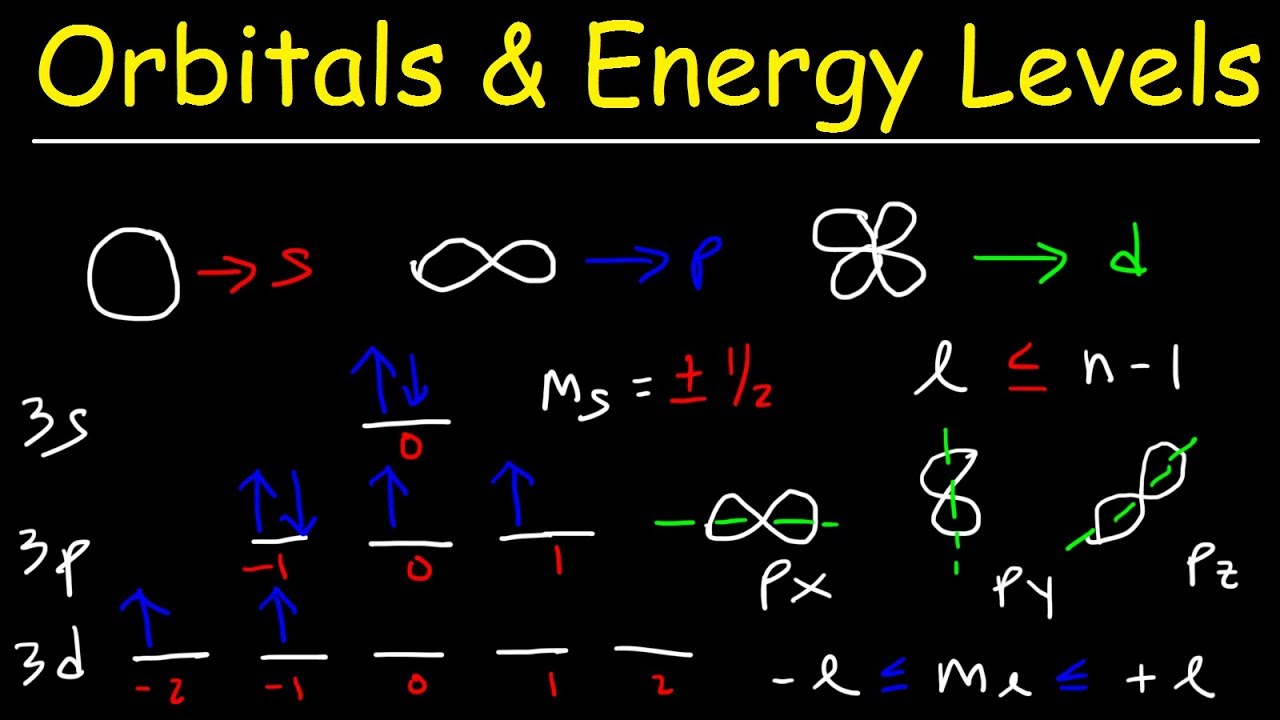
Spdf Orbitals : Parsing Spdf Orbital Hybridization And Simple Bonding

What is the maximum number of electrons that can occupy the 3d orbitals

Five d -orbitals in a cubic crystal field which split into two e g
